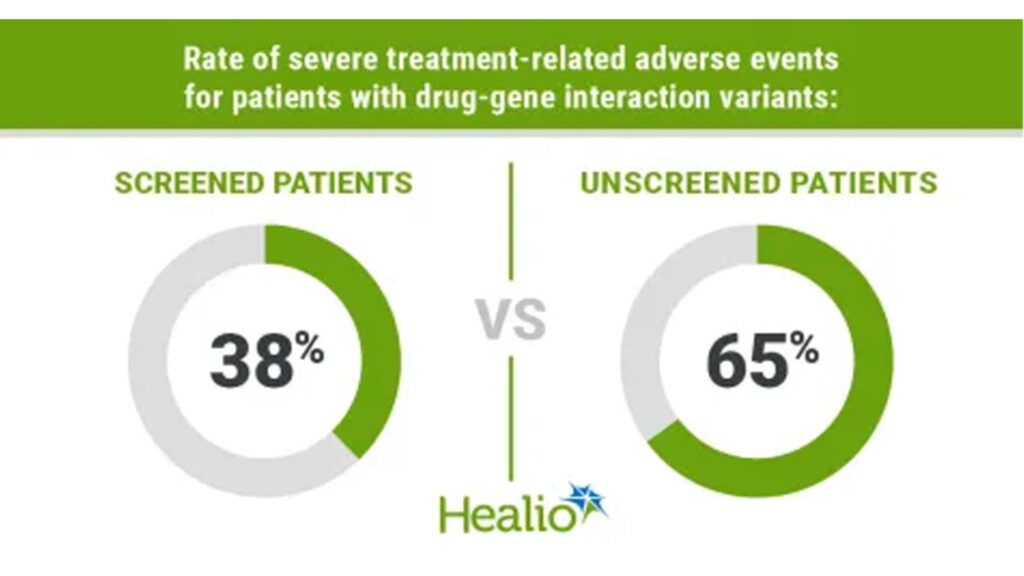
A prospective study led by Penn Medicine researchers shows that genetic screening for DPYD and UGT1A1 variants before chemotherapy can substantially reduce hospitalizations and ER visits from drug-related toxicities in gastrointestinal cancers. Median turnaround for results was 10 days, and more than half of patients had results before treatment. Despite NCCN only recently recommending that clinicians “consider” testing (Feb 2025), researchers urge broader adoption, citing both feasibility and patient safety.
Key Takeaways
- Actionable variants matter: 5–8% of patients carry clinically significant DPYD or UGT1A1 variants that affect drug metabolism.
- Preventable toxicity: Patients with known variants who received dose adjustments had fewer severe adverse events and treatment discontinuations.
- Infrastructure improvements: New workflows reduced testing time to ~7–10 days, integrated results into EHRs, and added clinical decision support alerts.
- Global momentum: EMA mandated DPYD testing in 2020; NCCN only added it as a consideration in 2025, but FDA labeling and guidelines could accelerate uptake.
- Future outlook: Broader pharmacogenomic sequencing beyond single genes may soon help tailor chemotherapy and supportive care drugs to each patient.