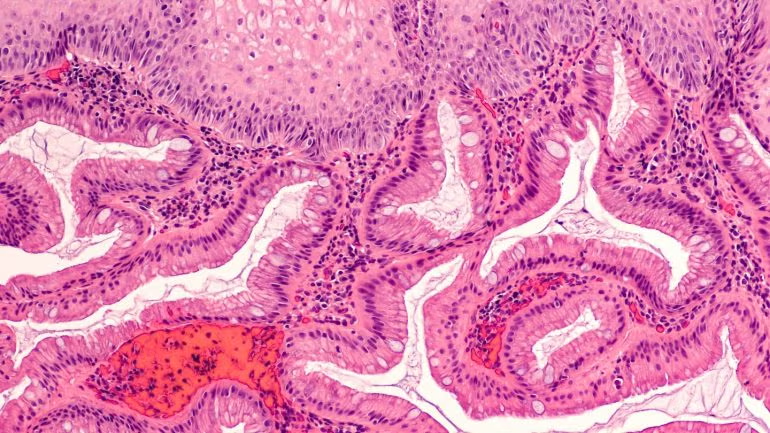
Cyted has secured $44 million to propel its FDA-cleared EndoSign capsule sponge into the US market, positioning the minimally invasive device as a potential game-changer in diagnosing Barrett’s oesophagus and related esophageal conditions. Unlike traditional endoscopy, the swallowable capsule dissolves in the stomach to collect samples, making early detection more accessible and scalable. Backed by EQT Life Sciences and other major investors, and with more than 35,000 tests already completed through the UK’s NHS, the company now aims to validate new biomarkers, expand its test portfolio, and reshape GI diagnostics in primary care. With US trials underway and pharma partnerships emerging, could EndoSign disrupt the entrenched endoscopy model and redefine screening for esophageal cancer risk?
Cyted raises $44m to propel US market expansion of its endoscopic capsule (Medical Device Network)
0