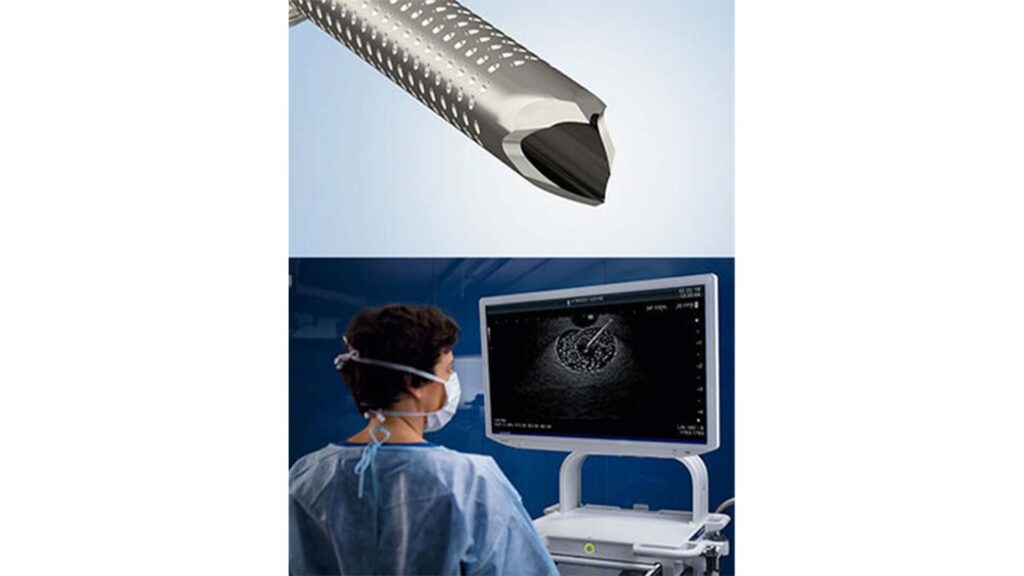
Olympus Corporation of the Americas has announced the U.S. launch of SecureFlex™, its newest single-use fine needle biopsy (FNB) device designed for endoscopic ultrasound–guided tissue sampling, particularly in challenging anatomies such as the pancreatic head and uncinate process.
The SecureFlex FNB needle is engineered to collect larger, intact tissue samples, addressing growing diagnostic needs in pancreatic cancer as molecular profiling and targeted therapies increasingly depend on high-quality histologic specimens. It is available in 19G, 22G, and 25G sizes.
Key design features include a dual-beveled cutting tip to preserve tissue architecture, nitinol construction to maintain needle integrity in tortuous anatomy, and enhanced ultrasound visibility to support precise targeting. Olympus positions the device as improving both sampling reliability and procedural efficiency during EUS-FNB.
Why it matters:
As precision oncology expands in GI malignancies, tissue quality—not just access—has become critical. Devices like SecureFlex reflect a broader shift in endoscopy toward tools optimized for downstream molecular testing, reinforcing EUS-FNB’s central role in pancreatic and hepatobiliary diagnostics.